vanadium electrons|vanadium electron dot diagram : Baguio Electrons are the permanent core particles of an atom. It resides in a specific orbit of the atom and revolves around the nucleus. The properties of the elements and their compounds depend on the electron configuration. . Tingnan ang higit pa Next Fifty Fifty Lottery FF-91 Draw on 10-04-2024. Tomorrow draw details. Karunya Plus KN 516 draw will hold at Gorkhy Bhavan on 04-04-2024. Kerala State Lotteries Results
vanadium electrons,When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion. The ionic properties of the elements depend on the exchange of electrons. In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change. . Tingnan ang higit paAn atom is the smallest particle of an element that has no independent existence but is directly involved in chemical . Tingnan ang higit paProtons are the permanent core particles of an atom. It resides in the center or nucleus of the atom. When a hydrogen atom removes an electron from its orbit, the . Tingnan ang higit paScientist Chadwick discovered neutrons in 1932. It is located in the nucleus at the center of the atom. The neutron is a charge-neutral particle and it is expressed by n. The charge of a neutron is zero and the . Tingnan ang higit pa

Electrons are the permanent core particles of an atom. It resides in a specific orbit of the atom and revolves around the nucleus. The properties of the elements and their compounds depend on the electron configuration. . Tingnan ang higit pa
Electrons are the permanent core particles of an atom. It resides in a specific orbit of the atom and revolves around the nucleus. The properties of the elements and their compounds depend on the electron configuration. . Tingnan ang higit paThe chemistry of vanadium is noteworthy for the accessibility of the four adjacent oxidation states 2–5. In an aqueous solution, vanadium forms metal aquo complexes of which the colors are lilac [V(H2O)6] , green [V(H2O)6] , blue [VO(H2O)5] , yellow-orange oxides [VO(H2O)5] , the formula for which depends on pH. Vanadium(II) compounds are reducing agents, and vanadium(V) comp.Vanadium(IV) has one unpaired 3d electron that, coupled with the nuclear spin, is exquisitely diagnostic in EPR spectroscopy - the vanadyl ion (VO 2+) is a sensitive . The arrangement of electrons in vanadium in specific rules in different orbits and orbitals is called the electron configuration of vanadium. The electron configuration of vanadium is [ Ar] 3d 3 4s 2 , if the electron . Electron configuration of Vanadium is [Ar] 3d3 4s2. Possible oxidation states are +2,3,4,5 . The chemistry of vanadium is noteworthy .Vanadium (V) has an atomic mass of 23. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.It has an atomic weight of 50.9415 and a mass number of 51. Vanadium has twenty-three protons and twenty-eight neutrons in its nucleus, and twenty-three electrons in four .vanadium electrons Vanadium's oxidation states. Vanadium has oxidation states in its compounds of +5, +4, +3 and +2. This section looks at ways of changing between them. .
Vanadium is a bright white, soft, ductile metal with good structural strength. Vanadium is resistant to attack by alkalis, hydrochloric acid, sulfuric acid, and salt water. When present in compounds, vanadium exists mostly in .
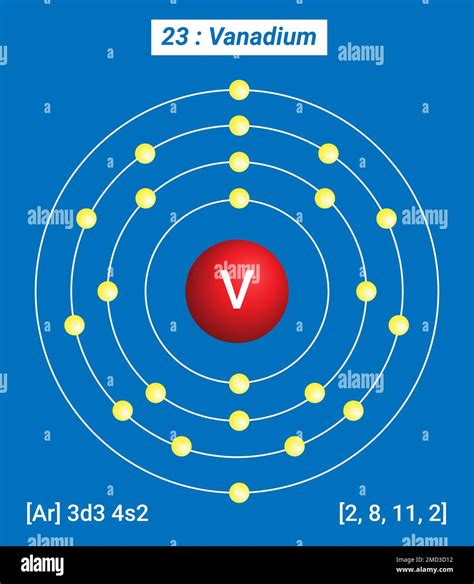
Vanadium atoms have 23 electrons and the shell structure is 2.8.11.2. The ground state electronic configuration of neutral vanadium is [ Ar ]. 3d3. 4s2 and the term symbol of .
The electron configuration of an atom describes the arrangement of electrons in its energy levels or shells. In the case of vanadium, its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d3. Vanadium has two valence electrons, which are the electrons in the outermost energy level.

icon : plus-circle. Protons et neutrons dans le Vanadium. Le vanadium est un élément chimique de numéro atomique 23, ce qui signifie qu’il y a 23 protons dans son noyau. Le nombre total de protons dans le noyau est appelé le numéro atomique de l’atome et reçoit le symbole Z.. La charge électrique totale du noyau est donc +Ze, où e (charge .
Atomic Number – Protons, Electrons and Neutrons in Vanadium. Vanadium is a chemical element with atomic number 23 which means there are 23 protons in its nucleus.Total number of protons in the nucleus is . Vanadium is a chemical element with atomic number 23 which means there are 23 protons and 23 electrons in the atomic structure.The chemical symbol for Vanadium is V. Electron Configuration and Oxidation States of Vanadium. Electron configuration of Vanadium is [Ar] 3d3 4s2. Possible oxidation states are +2,3,4,5. Electron Configuration Nous pouvons voir que le vanadium (V) a un numéro atomique de 23 dans le tableau périodique. Le nombre total d’électrons dans un atome de vanadium est donc de 23. Les termes « degré d’oxydation » et « valence » ne sont peut-être pas les mêmes, mais ils sont numériquement presque identiques.vanadium electron dot diagramName: Vanadium Symbol: V Atomic Number: 23 Atomic Mass: 50.9415 amu Melting Point: 1890.0 °C (2163.15 K, 3434.0 °F) Boiling Point: 3380.0 °C (3653.15 K, 6116.0 °F) Number of Protons/Electrons: 23 Number of Neutrons: 28 Classification: Transition Metal Crystal Structure: Cubic Density @ 293 K: 5.8 g/cm 3 Color: Silverish Atomic StructureFull electron configuration of vanadium: 1s2 2s2 2p6 3s2 3p6 3d3 4s2. titanium ← vanadium → chromium. Vanadium, complete electron configuration. Solution: Method 2. Locate the atom on the periodic table. Figure 1.9.1 1.9. 1: Periodic table of the elements with the location of vanadium (V) highlighted. (CC-BY-NC-SA; Kathryn A. Newton) Starting at hydrogen and the 1s subshell, read across each row of the periodic table until you get to your chosen element. Like most transition metals, vanadium exists in a wide range of oxidation states — most commonly from +2 to +5, but all states from −1 to +5 exist and even the rare −3 is known, in V (CO) 53 .vanadium electrons vanadium electron dot diagram The vanadium electron configuration, represented as [] 4s 2 3d 3 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 3, illustrates the precise arrangement of electrons within the atom.This configuration can be determined through various methods, including the aufbau principle, periodic table organization, Bohr model representation, or orbital diagram .The atomic number of Vanadium is 23, with an electron configuration of [Ar] 3d³ 4s². This configuration indicates that vanadium has three electrons in its 3d orbitals and two electrons in its 4s orbital. This unique configuration is responsible for vanadium's chemical reactivity and its ability to form multiple oxidation states.In the same manner of other transition metals, vanadium has added its electron to its inner shell. The configuration of 2-8-11-2 reflects the addition of three transition element electrons to the third shell. Vanadium's .
Here is a table of element valences. Remember that an element's electron cloud will become more stable by filling, emptying, or half-filling the shell. Also, shells don't stack neatly one on top of another, so don't always assume an element's valence is determined by the number of electrons in its outer shell.
Titanium has 22 protons, 26 neutrons and 22 electrons: 23: Vanadium has 23 protons, 28 neutrons and 23 electrons: 24: Chromium has 24 protons, 28 neutrons and 24 electrons: 25: Manganese has 25 protons, 30 neutrons and 25 electrons: 26: Iron has 26 protons, 30 neutrons and 26 electrons: 27: Cobalt has 27 protons, 32 neutrons and . It is helpful to first write down the electron configuration of an element at its ground state before attempting to determine the electron configuration of an element with an oxidation state. See examples below. Example 1: Vanadium; Vanadium at Ground State (Neutral): V: 5 d-electrons = [Ar] 4s 2 3d 3. Vanadium with an Oxidation State of +4: V .
Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.The Electron configuration of vanadium is [Ar]3d34s2. Vanadium is defined as a chemical element belonging to the periodic table, it is part of group 5, it is represented by the symbol V and its atomic number is 23. It is a rare metal, ductile and hard at the same time. It is found in different minerals and is basically used in various alloys. Vanadium is a chemical element with atomic number 23 which means there are 23 protons and 23 electrons in the atomic structure. The chemical symbol for Vanadium is V . Vanadium is a hard, silvery grey, ductile, and malleable transition metal.
vanadium electrons|vanadium electron dot diagram
PH0 · vanadium electron dot diagram
PH1 · vanadium electron diagram
PH2 · vanadium electron configuration long form
PH3 · vanadium electron configuration ground state
PH4 · vanadium electron configuration diagram
PH5 · vanadium electron configuration
PH6 · valence electrons vanadium
PH7 · v2o5 price
PH8 · Iba pa